Hi
Some people have been using this product in the Netherlands and it said to be revolutionary. I had a look at their website and found some interesting stuff.
Link: http://www.festal.nl/shop/index.php?action=extra&extra=A_unieke_meststof_uitgelicht&lang=NL (use Google Translate on the website to convert it to English)
They claim they use an optimal balance between elements so antagonism does not occur. But if I recall correctly, this antagonism effect is based on terrestrial systems and not aquatic systems?
And what about this (beware, translated by Google!) : "In particular, the very important for the leaf quality, together with the Calcium sulphate, phosphate and bicarbonate, insoluble compounds. for which the plant is not recordable!" ?
I didn't know there was a reaction between CaSO4 and PO4. The say they use a "special" type of Calcium. Can anyone elaborate on this?
Here's the breakdown of their fert: https://lh6.googleusercontent.com/-jIYkrf-p77Y/UA2qBueGvII/AAAAAAAACXQ/YgSL9SbBJlU/s800/IMG_8146.JPG
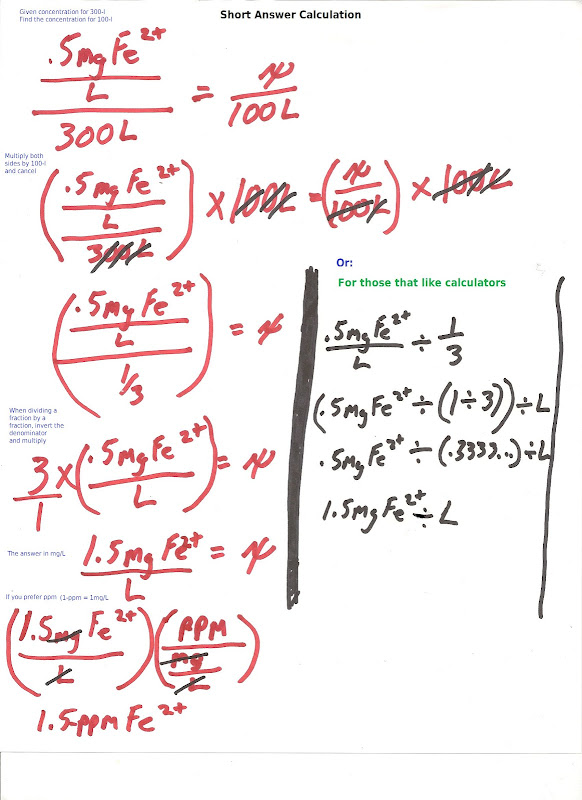
(They advise to dose 5ml of this weekly on 100 liter)
Cheers,
Gilles
Some people have been using this product in the Netherlands and it said to be revolutionary. I had a look at their website and found some interesting stuff.
Link: http://www.festal.nl/shop/index.php?action=extra&extra=A_unieke_meststof_uitgelicht&lang=NL (use Google Translate on the website to convert it to English)
They claim they use an optimal balance between elements so antagonism does not occur. But if I recall correctly, this antagonism effect is based on terrestrial systems and not aquatic systems?
And what about this (beware, translated by Google!) : "In particular, the very important for the leaf quality, together with the Calcium sulphate, phosphate and bicarbonate, insoluble compounds. for which the plant is not recordable!" ?
I didn't know there was a reaction between CaSO4 and PO4. The say they use a "special" type of Calcium. Can anyone elaborate on this?
Here's the breakdown of their fert: https://lh6.googleusercontent.com/-jIYkrf-p77Y/UA2qBueGvII/AAAAAAAACXQ/YgSL9SbBJlU/s800/IMG_8146.JPG
(They advise to dose 5ml of this weekly on 100 liter)
Cheers,
Gilles
Last edited by a moderator: