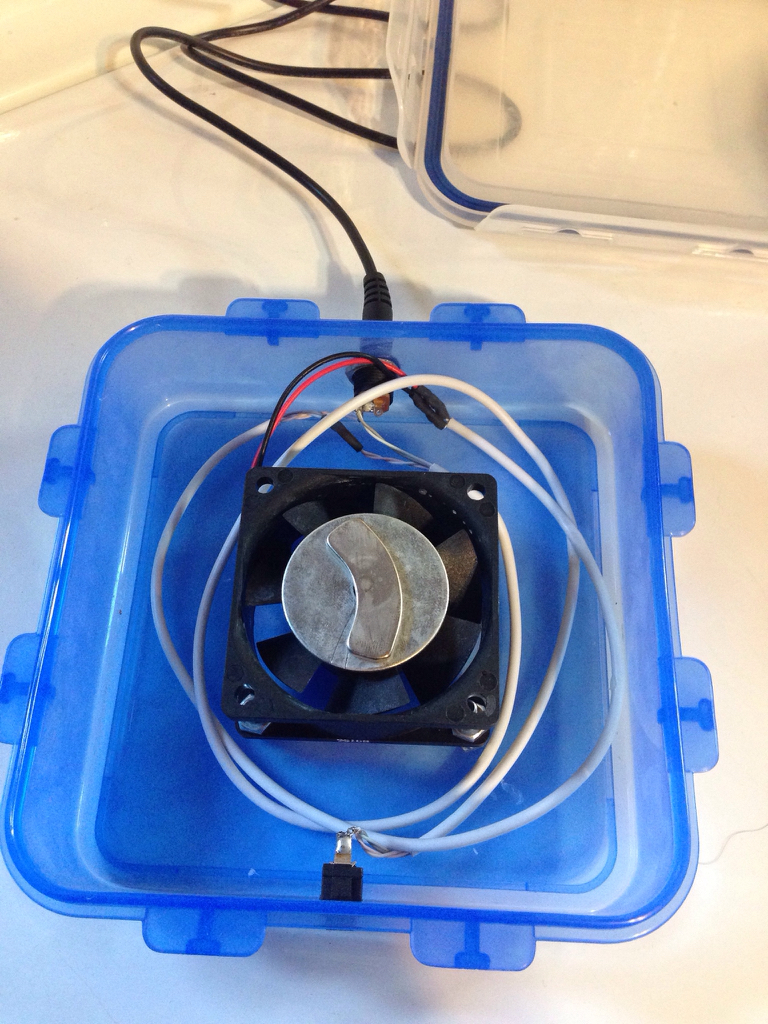
For anyone that is using an auto doser, do you employ some sort of auto stirring mechanism to keep the ferts suspended?
I have been auto dosing ferts for a few years now using a Neptune DOS. It occurred to me that when I first mix up the ferts for my dosing containers i get lack luster plant growth and then towards the end of the dosing container i start getting better plant growth. There is a lot of settling that occurs and sits on the bottom of the dosing containers. My thought is if i employ a magnetic mixer or some kind of micro pump in the dosing container that stirs the mix every day before it gets pumped into the water column i might get more consistent growth.
For reference I employ the PPS Pro method and have 2 separate dosing containers. One with Micros and one with Macros.
Does anyone have any experience with employing some sort of stirrer?
I have been auto dosing ferts for a few years now using a Neptune DOS. It occurred to me that when I first mix up the ferts for my dosing containers i get lack luster plant growth and then towards the end of the dosing container i start getting better plant growth. There is a lot of settling that occurs and sits on the bottom of the dosing containers. My thought is if i employ a magnetic mixer or some kind of micro pump in the dosing container that stirs the mix every day before it gets pumped into the water column i might get more consistent growth.
For reference I employ the PPS Pro method and have 2 separate dosing containers. One with Micros and one with Macros.
Does anyone have any experience with employing some sort of stirrer?